I needed to prepare a plasmid library and was curious which had the best yield
Bottom line: they are about the same for two component assembly
- By gel both rxns are about 25% efficiency
- Both rxns have about 3ng/uL backbone/vector and yield about 0.75ng/uL of plasmid
- They gave a similar number of colonies when transformed
Experiment
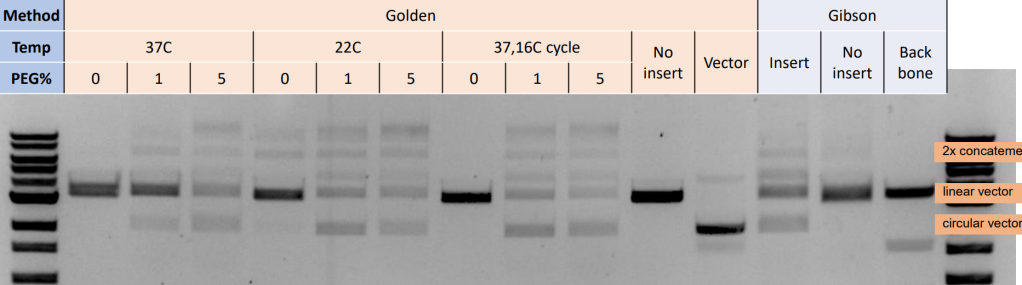
- Both were used to make the same target plasmid
- Both were two component assembly rxns (~3550 bp backbone + ~100bp insert)
- Both rxns had ~3ng/uL backbone
- Loaded 10uL rxn in each well
- Gibson – used 20bp overlaps and an
- Used homemade Gibson MM which is amount 2x better than NEB HiFi (both the paper and my independent verification )
- Reference for homemade Gibson: Rabe et al. (2020) A Simple Enhancement for Gibson Isothermal Assembly
- Vector prepared by PCR, insert was 87nt ssDNA, 20bp overlaps
- Used NEB enzymes
- Reacted for 1hr
- Golden
- All reactions were run overnight
- cycling: 5 min 37°C → 5 min 16°C for 12 hours
- final 60C 5min heat treatment
- Insert prepared by PCR
- Vector was plasmid with 2 BsaI sites
- Used NEB enzymes/buffer
Conclusions
- Gibson and Golden have roughly the same efficiency (compare E with L)
- Enhancer (PEG, extra ATP, BSA) makes a big difference on golden gate rxns. I’m not sure which of these components are the important parts.
- Golden works equally well with cycling or 22C overnight
Golden Gate details
| REAGENTS | Stock Conc | Rxn (uL) | Final |
| Destination Plasmid3588bp | 260ng/uL | 0.12 | 3ng/uL |
| T4 DNA Ligase Buffer (with ATP) | 5x | 2.0 | |
| H2O | 3.1 | ||
| T4 DNA Ligase | 400 U/µl | 0.5 | 20U/uL |
| BsaI-HFv2 | 20 U/µl | 0.3 | 0.6U/uL |
| Insert (VR127-1C)109bp | 0.9ng/uL | 2.0 | 0.18ng/uL (2 molar eq) |
| 5x ligation enhancer | 2.0 | ||
| Total | 10 |
Gibson Details
| Reagent | Quantity (uL) | |
| 2x homemade Gibson | 5.00 | |
| Backbone (VR119-1N) 20ng/uL | 1.88 | 3.7ng/uL |
| Water | 1.88 | |
| Insert ssDNA(5ng/uL) | 1.25 | 0.63ng/uL |
| Total | 10.0 |
Leave a comment