Instructions
Use 0.2x (final) SYBR green in your qPCR mix.
Notes
- You can do everything else as usual (PCR rxn setup and cycling parameters)
- SYBR green typically comes as a 10,000x stock in DMSO. You can dilute 1uL of 10,000x in 625uL of water and add 0.25uL of this dilution per 20uL qPCR rxn.
- I have done dozens of qPCR rxns with Taq, Phusion, AccuPrime and 0.2X SYBR and it has always worked.
Evidence
- Added serial dilution of SYBR green to determine the optimal amount
- Used NEB Taq and followed their procedure
Conclusions: Too much SYBR >0.33x results in no amplification. I picked 0.2x as a safe amount to add.
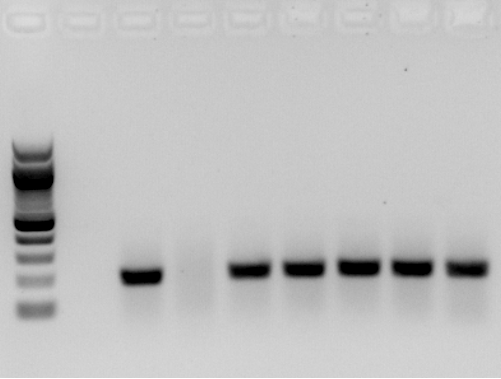
| Lane | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| SYBR green (x) | 100bp ladder | 0 (no template) | 0 | 1.3 | 0.33 | 0.08 | 0.02 | 0.05 | 0.0013 |
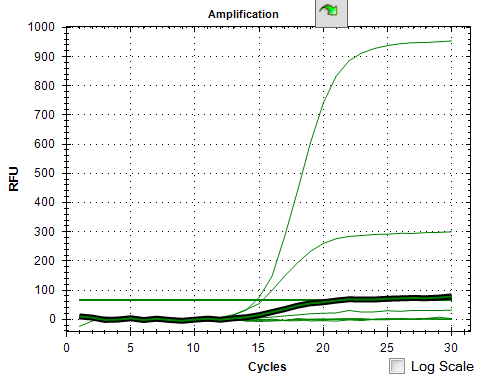
PCR Details for this experiment
| Component | 20 μl rxn | Final Concentration |
| 10X Standard Taq Reaction Buffer | 2.0 | 1X |
| 10 mM dNTPs | 0.4 | 200 µM |
| Taq DNA Polymerase | 0.1 | 1.25 units/50 µl PCR |
| Water | 15.7 | |
| 5 µM each primer (165&166) | 0.8 | 0.4 µM (0.05–1 µM) |
| SYBR GreenStock: 300x dilution | varies | varies |
| Template DNAE coli gDNA (550ng/uL) | 0.36 | 10ng/uL |
| STEP | TEMP | TIME |
| Initial Denaturation | 95°C | 30 seconds |
| 30 Cycles | 95°C 50°C 68°C | 15 seconds 15 seconds 20 seconds |
Leave a comment